Your Partner in EMS
group000550
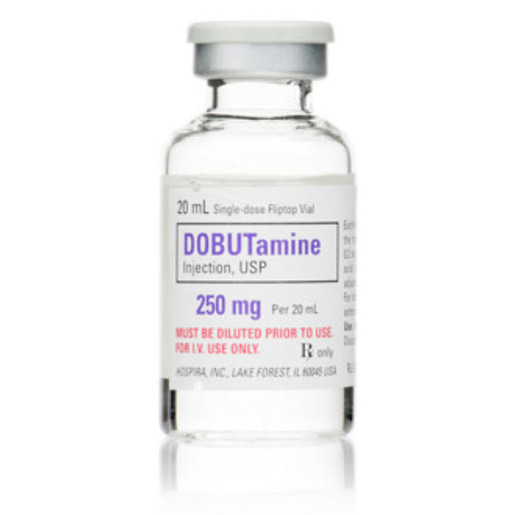
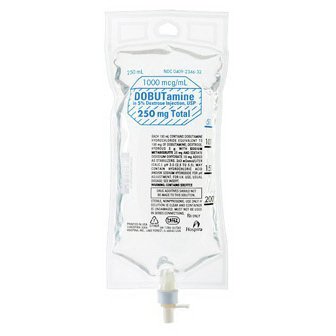
Dobutamine
Manufacturer:
PFIZER INC.
Regulated product
Non-Returnable Product
Manufacturer:
PFIZER INC.
Due to manufacturer requirements, this product is no longer available to be ordered in the EACH quantity. Learn more.
Always follow your local protocols regarding product use.
➤ License Authorization Form (LAF) Required
Please note: These items are available from multiple manufacturers including Hospira Worldwide, Meridian Medical Technologies and Other Manufacturers
Added to Your Shopping Cart
Refine results
No Filters Available
Are you sure you want to clear this supply list?

|
|
|---|---|
| Product # | 2346-32 |
| Color | Colorless |
| Life Stage | 250mL |
| Application Type | Human Prescription Drug |
| NDC | 0409-2346-32 |
| Sterile | Sterile |
| Latex Free | Yes |
| Uses | Dobutamine in 5% Dextrose Injection, USP is Indicated when Parenteral Therapy is Necessary for Inotropic Support in the Short-Term Treatment of Patients with Cardiac Decompensation Due to Depressed Contractility Resulting Either from organic Heart Disease or from Cardiac Surgical Procedures |
| Active Ingredient | Dobutamine, Hydrochloride |
Related Products

Item #:
8600-STB001B
Curaplex® Stop the Bleed®, Basic Kit with C-A-T
By:
CURAPLEX
Your Price:
Log in
List Price:
$79.99 EA
Log in for availability
Item #:
View Multiple
Curaplex® GO-PAP™ Capno Kits
By:
CURAPLEX
Your price:
Log in for sale price
List Price: from $112.99 EA
Log in for availability

Item #:
8600-STB003A
Curaplex® Stop the Bleed®, Advanced Kit with C-A-T
By:
CURAPLEX
Your Price:
Log in
List Price:
$251.99 EA
Log in for availability

Item #:
670159-KIT
Curaplex® Stop the Bleed® Multi Pack Kit, Basic
By:
CURAPLEX
Your Price:
Log in
List Price:
$410.99 EA
Log in for availability

Item #:
1715-01837
Curaplex® Basic Blood Transfusion Kit
By:
CURAPLEX
Your Price:
Log in
List Price:
$90.99 EA
Log in for availability

Item #:
STB001B-XT
Curaplex® Stop the Bleed®, Basic With SAM XT
By:
CURAPLEX
Your Price:
Log in
List Price:
$76.79 EA
Log in for availability

Item #:
View Multiple
Curaplex® NIO® Kits
By:
CURAPLEX
Your Price:
Log in
List Price: from $159.99 EA
Log in for availability

Item #:
View Multiple
Curaplex® Tactical Field Suction Easy Kits
By:
CURAPLEX
Your Price:
Log in
List Price: from $86.99 EA
Log in for availability
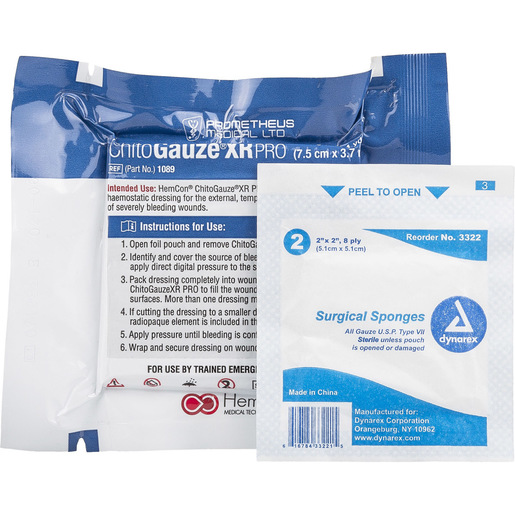
Item #:
670138-KIT
Chitogauze Kit
By:
CURAPLEX
Your Price:
Log in
List Price:
$61.99 EA
Log in for availability

Item #:
STB001B-SOFTT
Curaplex® Stop The Bleed®, Basic Kit With SOFT-T
By:
CURAPLEX
Your Price:
Log in
List Price:
$82.99 EA
Log in for availability