
Your Partner in EMS
group001762
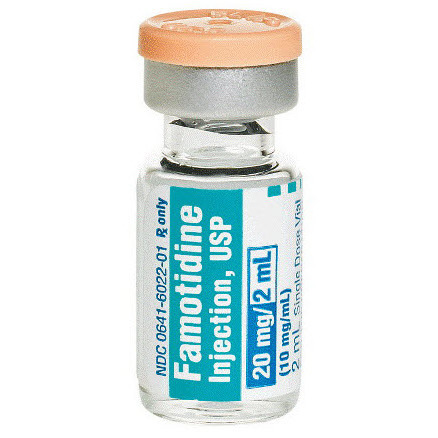
Famotidine
Manufacturer:
OTHER MANUFACTURER
Regulated product
Non-Returnable Product
Manufacturer:
OTHER MANUFACTURER
Always follow your local protocols regarding product use.
Added to Your Shopping Cart
Refine results
No Filters Available
Are you sure you want to clear this supply list?
