Your Partner in EMS
group005503
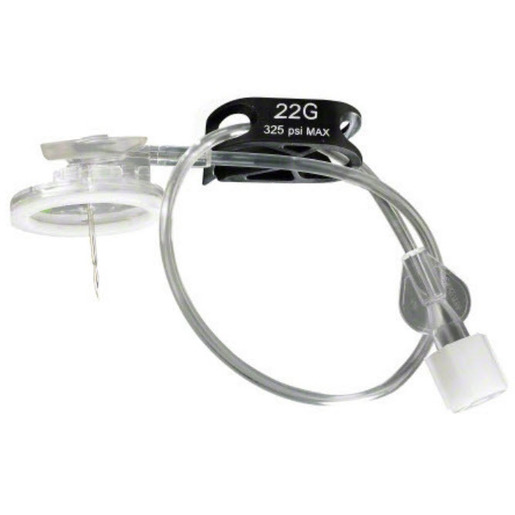
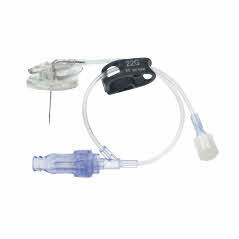
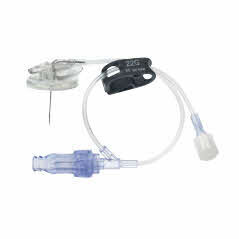
Surecan™ Safety II Port Access Needles
Manufacturer:
B. BRAUN MEDICAL INC.
Manufacturer:
B. BRAUN MEDICAL INC.
The Surecan™ Safety II Port Access Needle, a safety engineered needle for accessing implanted intravenous ports. The non-coring and angled needles are suitable for power injections up to 325 psi.1 The Surecan Safety II has a manually activated safety mechanism that is designed to deploy upon needle removal and to shield the tip, to reduce needlestick injuries.
Features:
- A green dot, which appears through the clear bottom plate when the safety mechanism is successfully engaged.
- A low profile and foam pad, designed to assist with the placement of a securement dressing. The nonabsorbent pad and closed cell materials are designed to help reduce the risk of needlestick injuries.
- Flexible and ergonomic wings, provide secure handling during insertion and removal of the needle.2
- A small, transparent, round based plate, designed to help increase stability and help visualize the placement of the needle.
The Surecan Safety II is available with or without a Y-site and pre-attached CARESITE® luer access device.
Added to Your Shopping Cart
Refine results
Color
Needle Length
Needle Size
Are you sure you want to clear this supply list?

|

|

|

|

|

|
|
|---|---|---|---|---|---|---|
| Product # | 1611-01002 | 1612-70602 | 1612-00702 | 262319 | 1611-50002 | 1811-52002 |
| Color | Brown | Yellow | Yellow | Black | Yellow | Yellow |
| Material | Polyurethane, Stainless Steel | Polyurethane, Stainless Steel | Polyurethane, Stainless Steel | Polyurethane, Stainless Steel | Polyurethane, Stainless Steel | Polyurethane, Stainless Steel |
| Sterile | Sterile | Sterile | Sterile | Sterile | Sterile | Sterile |
| Needle Length | 0.8in | 0.8in | 1in | 0.8in | 0.6in | 1in |
| Tip Type | Beveled | Beveled | Beveled | Beveled | Beveled | Beveled |
| Use Frequency | Single Use | Single Use | Single Use | Single Use | Single Use | Single Use |
| Disposable | Disposable | Disposable | Disposable | Disposable | Disposable | Disposable |
| Latex-free | Latex-free | Latex-free | Latex-free | Latex-free | Latex-free | Latex-free |
| Needle Size | 19ga | 20ga | 20ga | 22ga | 20ga | 20ga |
Related Products

Item #:
View Multiple
Curaplex® SAM® IO Needle Kits
By:
CURAPLEX
Your Price:
Log in
List Price: from $94.99 EA
Log in for availability

Item #:
View Multiple
ARS® Needle Decompression Kits
By:
NORTH AMERICAN RESCUE, LLC
Your Price:
Log in
List Price: from $14.99 EA
Log in for availability
Item #:
View Multiple
SAM® IO Needles
By:
SAM MEDICAL
Your Price:
Log in
List Price: from $89.99 EA
Log in for availability

Item #:
View Multiple
Jamshidi Intraosseous (IO) Needles
By:
BECTON DICKINSON
Your Price:
Log in
List Price: from $62.59 EA
Log in for availability

Item #:
View Multiple
SafetyGlide™ Needles
By:
BECTON DICKINSON
Your Price:
Log in
List Price: from $0.89 EA
Log in for availability
Item #:
View Multiple
Luer Tip Syringes without Needles
By:
B. BRAUN MEDICAL, INC
Your Price:
Log in
List Price: from $0.19 EA
Log in for availability

Item #:
View Multiple
Safetyglide Needles w/Syringe
By:
BECTON DICKINSON
Your Price:
Log in
List Price: from $63.99 BX of 50 EA
Log in for availability

Item #:
View Multiple
BD Eclipse Safety Needles
By:
BECTON DICKINSON
Your Price:
Log in
List Price: from $63.99 BX of 100 EA
Log in for availability
Item #:
View Multiple
Hypodermic Needle-Pro® w/ Safety Needles
By:
SMITHS MEDICAL ASD, INC.
Your Price:
Log in
List Price: from $48.79 BX of 100 EA
Log in for availability

Item #:
View Multiple
Dynarex Syringes without Needles
By:
DYNAREX CORPORATION
Your Price:
Log in
List Price: from $17.49 BX of 100 EA
Log in for availability