Your Partner in EMS
group001828
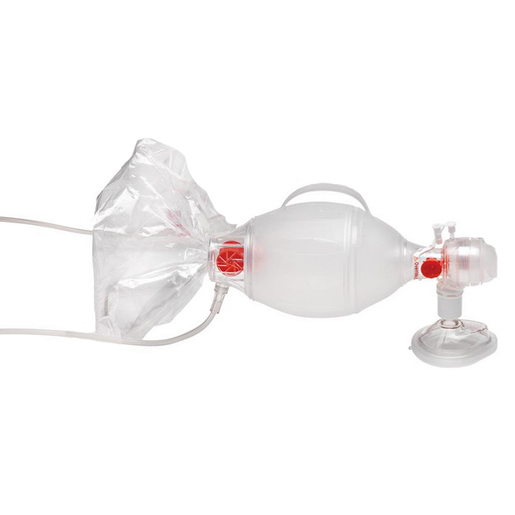
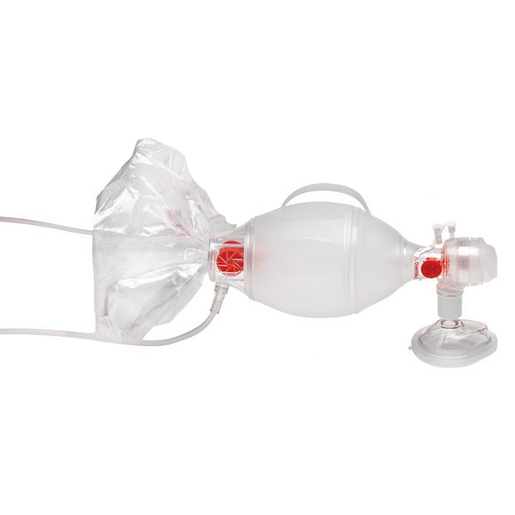
Ambu® SPUR® II BVM, Pediatric
Manufacturer:
AMBU INC.
Manufacturer:
AMBU INC.
Ambu® SPUR® II manual resuscitator (BVM) provides users with exceptional tactile and visual feedback during resuscitation. The bag is highly responsive, with minimal mechanical resistance. Furthermore the characteristic design provides optimum stroke volume with perfect recoil. Ambu Spur II is MR conditional and can be used in the MRI environment according to the following conditions:
- Static magnetic field of 3-Tesla or less
- Spatial gradient magnetic field of 720-Gauss/cm or less
Ambu SPUR II is not intended to be in direct contact with a patient undergoing an MRI procedure.
- Single-shutter valve system for reliable functionality
- Integrated handle for user comfort and uniform compression
- Thin-walled compression bag allows for lung compliance and “feel”
- SafeGrip™ surface for secure handling in stressful environments
- M-Port allows sidestream measuring of etCO2 or quick medication delivery without disconnecting the Ambu SPUR II from the ET tube
- Easy attachment of manometer and PEEP valve MR conditional
- Pediatric max tidal volume is approx. 450 ml
- Bag reservoir volume is approx. 2600 ml
- Patient connector: 22/15 mm (IS0)
- Expiratory connector: 30 mm
Added to Your Shopping Cart
Refine results
Life Stage
Are you sure you want to clear this supply list?

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|

|

|

|

|

|

|

|

|

|

|
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Product # | 065-530616000EA | 2442-21403 | 2442-33014 | 2442-83014 | 2442-21402 | 2442-614 | 2442-01702 | 2442-21400 | 065-530212000EA | 2442-00002 | 065-530-212-001 | 065-530-612-001 | 2442-05401 | 2442-213 | AU530213033 | 2442-13031 | 2442-53231 | 065-531-613-000 | 2442-03052 | 530-213 | 2442-05405 | 2442-01705 | 2442-53213 | 2442-80020 | 2442-03005 | 2442-21502 |
| Material | SEBS Polymer | SEBS Polymer | SEBS Polymer | SEBS Polymer | SEBS Polymer | SEBS Polymer | SEBS Polymer | SEBS Polymer | SEBS Polymer | SEBS Polymer | SEBS Polymer | SEBS Polymer | SEBS Polymer | SEBS Polymer | SEBS Polymer | SEBS Polymer | SEBS Polymer | SEBS Polymer | SEBS Polymer | SEBS Polymer | SEBS Polymer | SEBS Polymer | SEBS Polymer | SEBS Polymer | SEBS Polymer | SEBS Polymer |
| Life Stage | Neonate and Infant | Neonate, Infant and Toddler | Neonate, Infant and Toddler | Neonate, Infant and Toddler | Neonate, Infant and Toddler | Neonate, Infant and Toddler | Neonate, Infant and Toddler | Neonate, Infant and Toddler | Infant | Infant | Infant | Infant | Infant | Toddler | Pediatric | Toddler | Toddler | Toddler | Toddler | Toddler | Toddler | Toddler | Toddler | Toddler | Toddler | Infant and Toddler |
| Disposable | Disposable | Disposable | Disposable | Disposable | Disposable | Disposable | Disposable | Disposable | Disposable | Disposable | Disposable | Disposable | Disposable | Disposable | Disposable | Disposable | Disposable | Disposable | Disposable | Disposable | Disposable | Disposable | Disposable | Disposable | Disposable | Disposable |
| Latex-free | Latex-free | Latex-free | Latex-free | Latex-free | Latex-free | Latex-free | Latex-free | Latex-free | Latex-free | Latex-free | Latex-free | Latex-free | Latex-free | Latex-free | Latex-free | Latex-free | Latex-free | Latex-free | Latex-free | Latex-free | Latex-free | Latex-free | Latex-free | Latex-free | Latex-free | Latex-free |
Documents
Related Products

Item #:
View Multiple
Curaplex® Select BVM
By:
CURAPLEX
Your Price:
Log in
List Price: from $29.79 EA
Log in for availability

Item #:
8600-01487
Curaplex® VT Select BVM Kit
By:
CURAPLEX
Your Price:
Log in
List Price:
$55.99 EA
Log in for availability

Item #:
670250-KIT
Curaplex® BVM with Filterline Kit
By:
CURAPLEX
Your Price:
Log in
List Price:
$43.99 EA
Log in for availability

Item #:
670251-KIT
Curaplex® Pediatric BVM with Filterline Kit
By:
CURAPLEX
Your Price:
Log in
List Price:
$56.99 EA
Log in for availability

Item #:
View Multiple
Ambu® SPUR® II BVM, Adult
By:
AMBU INC.
Your price:
Log in for sale price
List Price: from $16.29 EA
Log in for availability

Item #:
View Multiple
The Pocket BVM™
By:
SAFEGUARD MEDICAL
Your Price:
Log in
List Price: from $77.99 EA
Log in for availability
Item #:
View Multiple
Ambu® SPUR® II BVM, Infant
By:
AMBU INC.
Your Price:
Log in
List Price: from $23.79 EA
Log in for availability

Item #:
View Multiple
Airflow™ Manual Resuscitators BVM, Small Adult
By:
AIRLIFE
Your Price:
Log in
List Price: from $28.79 EA
Log in for availability

Item #:
2442-01910
Cyclone® Pocket BVM w/ Hard Olive Case
By:
NORTH AMERICAN RESCUE, LLC
Your Price:
Log in
List Price:
$92.99 EA
Log in for availability

Item #:
View Multiple
Airflow™ Manual Resuscitators BVM, Pediatric
By:
AIRLIFE
Your Price:
Log in
List Price: from $24.49 EA
Log in for availability