Your Partner in EMS
1812-11509
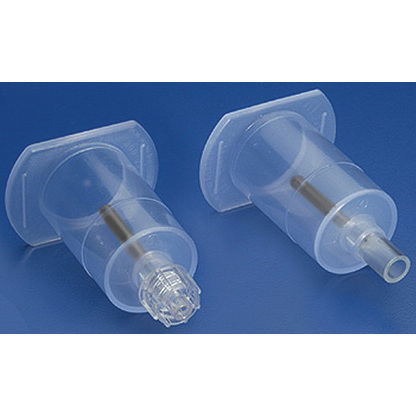
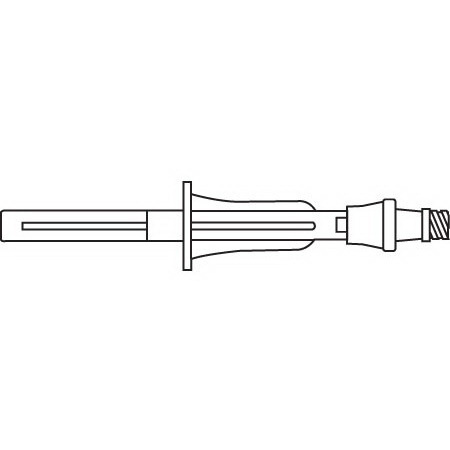
Lifeshield® Clave® IV Bag Access Device, 3-1/2in
Manufacturer:
ICU MEDICAL
Manufacturer:
ICU MEDICAL
LifeShield® Clave® I.V. bag access device - for attachment to a flexible IV container to allow needleless intermittent withdrawal of solution through a swabable luer-activated valve.
- Latex-free
- Non-DEHP
California Proposition 65 Warning:
⚠ WARNING: These products can expose you to chemicals such as vinyl chloride, ethylene oxide, bisphenol-A (BPA), diisodecyl phthalate (DIDP), and/or di(2-ethylhexyl) phthalate (DEHP) which are known to the State of California to cause cancer and/or birth defects or other reproductive harm. For more information, go to www.P65Warnings.ca.gov.
The above warning is required under California’s Safe Drinking Water and Toxic Enforcement Act of 1986, more commonly known as “Prop 65”. Some ICU products may be sterilized using ethylene oxide and some ICU intravenous tubing and drip chambers are manufactured using polyvinyl chloride (PVC) that may contain vinyl chloride, DIDP and/or DEHP. Also, certain manifold, stopcock, connector, and check valve products are manufactured using polycarbonates that may contain BPA. This shipment may contain one or more of these products.
Added to Your Shopping Cart
Are you sure you want to clear this supply list?

|
|
|---|---|
| Product # | 1812-11509 |
| Sterile | No |
| Disposable | Yes |
| Dimensions | 3-1/2in |