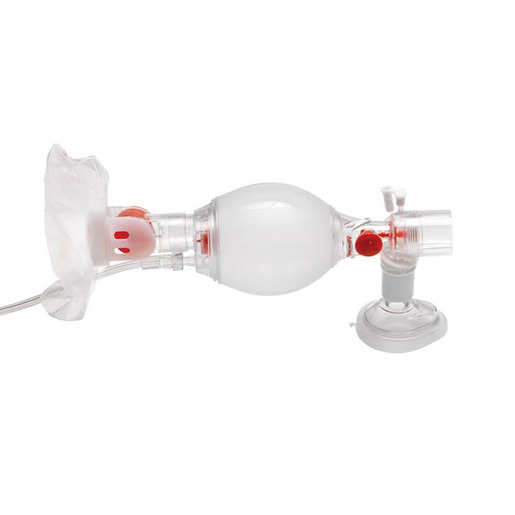
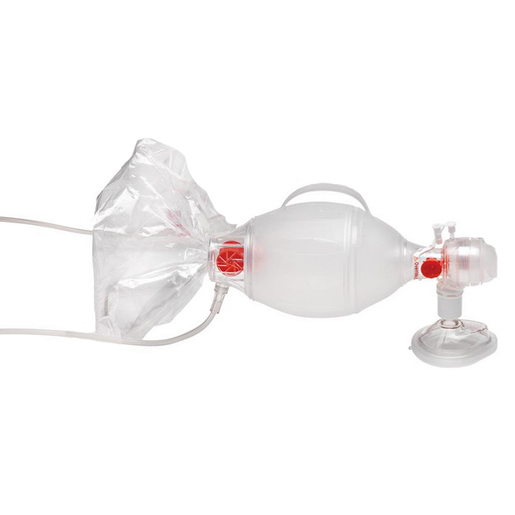
Ambu® Pediatric SPUR® II BVM Resuscitators with Pediatric Mask
Ambu® SPUR® II Disposable Resuscitator is the only single use resuscitator that is made from a SEBS polymer instead of PVC. This classifies Ambu® SPUR® II as environmentally safe and fully disposable, thus eliminating all risk of cross contamination. It is part of a complete family of resuscitators, including infant, pediatric, and adult sizes.
Ambu® SPUR® II provides users with exceptional tactile and visual feedback during resuscitation. The bag is highly responsive, featuring minimal mechanical resistance. Moreover, the characteristic design provides the optimum in respect to stroke volume and recoil. All Ambu® SPUR® II resuscitators come in individual, resealable carrying bags, complete with one or more masks and any special accessories - color-coded for fast size identification.
Features:
- SafeGrip surface for secure handling in stressful environments
- Ergonomic, lightweight design makes extended ventilations less fatiguing
- Fast recoil time allows for rapid ventilation
- Easy attachment of manometer and PEEP valve
- Unique single-shutter valve system for reliable functionality
- Swivel between valve and mask permits 360°C positioning in relation to the patient
- Integrated handle for user comfort and uniform compression
- Thin-walled compression bag allows for lung compliance and “feel”
- Extremely low valve resistance for unimpeded airflow
- Soft splashguard for user safety
Product photos may vary from actual product models, sizes and/or colors. Contact your account manager with any questions.
Are you sure you want to clear this supply list?

|
|
|---|---|
| Product # | 2442-13048 |
| Patient Size | Child |
| Reservoir Bag | Reservoir Bag |
| Manometer | Does Not Include Manometer |
| Disposable | Disposable |
| Latex-free | Latex-free |
| Filter | Does Not Include Filter |
| Tubing | O2 Tubing |
| Volume | 450mL |
| Material | SEBS Polymer |
| Life Stage | Pediatric |
| Valve | PEEP Valve |
| Mask Size | Child |
| Mask | Includes Mask |