
Your Partner in EMS
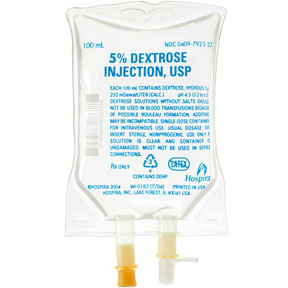
7923-23EA
Dextrose 5%, 100mL Bag
By:
ICU MEDICAL
Regulated product
By:
ICU MEDICAL
⚠ WARNING: These products can expose you to chemicals such as vinyl chloride, ethylene oxide, bisphenol-A (BPA), diisodecyl phthalate (DIDP), and/or di(2-ethylhexyl) phthalate (DEHP) which are known to the State of California to cause cancer and/or birth defects or other reproductive harm. For more information, go to www.P65Warnings.ca.gov.
Dextrose 5%, 100mL Flexible Container
Features:
- Single dose container
- NDC#: 0409-7923-23
- Prescribing information
Always follow your local protocols regarding product use.
⚠ License Authorization Form Required
This product is a prescription drug which requires a Medical Director or Pharmacist-in-Charge to authorize any purchases.
Download the License Authorization Form.
Added to Your Shopping Cart
Are you sure you want to clear this supply list?
|
|
|---|---|
| Product # | 7923-23EA |
| Volume | 100mL |
| Refrigeration | Refrigeration Not Required |
| NDC | 0409-7923-23 |
| Concentration | 5% |
| Type | Bag |
Related Products

Item #:
1911-01911EP
Acetaminophen, 10mg/mL, 100mL Premixed Bag
By:
B. BRAUN MEDICAL INC.
Your Price:
Log in
List Price:
$22.99 EA
Log in for availability

Item #:
View Multiple
G3+ Responder Bag
By:
STATPACKS, INC.
Your Price:
Log in
List Price: from $435.99 EA
Log in for availability

Item #:
602324X
Lactated Ringers, 1000mL Bag
By:
BAXTER HEALTHCARE-DMG
Your Price:
Log in
List Price:
$11.99 EA
Log in for availability

Item #:
LA3255
L.A. Rescue® O2 To Go Pro Plus Bag
By:
L.A. RESCUE LLC
Your Price:
Log in
List Price:
$228.99 EA
Log in for availability
Item #:
2521-87060
i-Gel® O2 Resus EMS Bag
By:
INTERSURGICAL, INC.
Your Price:
Log in
List Price:
$186.99 EA
Log in for availability

Item #:
View Multiple
Omni™ Pro X BLS/ALS Emergency Response Bags
By:
MERET
Your Price:
Log in
List Price: from $449.99 EA
Log in for availability
Item #:
602323
Lactated Ringers, 500mL Bag
By:
BAXTER HEALTHCARE-DMG
Your Price:
Log in
List Price:
$8.99 EA
Log in for availability

Item #:
684015RB
Curaplex® Pediatric ALS Bag
By:
CURAPLEX
Your Price:
Log in
List Price:
$499.99 EA
Log in for availability

Item #:
357953
Lactated Ringers, 1000mL Bag
By:
ICU MEDICAL
Your Price:
Log in
List Price:
$11.49 EA
Log in for availability

Item #:
1071-17367
Curaplex® Multi-Purpose Collection Bag with Hook
By:
CURAPLEX
Your Price:
Log in
List Price:
$27.99 PK of 12 EA
Log in for availability
